A) proton transfer
B) loss of leaving group
C) nucleophilic attack
D) rearrangement
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the most likely structure of the following cation after it has rearranged. 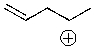
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is hydride?
A) H+
B) H
C) H−
D) H2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
Does a reaction with a positive ∆S and a negative ∆H favor reactants or products?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What pattern of curved arrow pushing is the second step of this reaction? 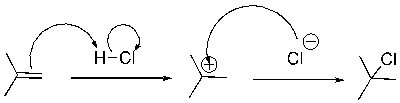
A) proton transfer
B) loss of leaving group
C) nucleophilic attack
D) rearrangement
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the nucleophilic atom in the following molecule, Me3P.
A) P
B) C
C) H
D) Me
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Melphalan, a drug used in chemotherapy, reacts with itself in the body before binding with its target, as illustrated in the mechanism below. Which two patterns of arrow pushing are seen in this reaction? 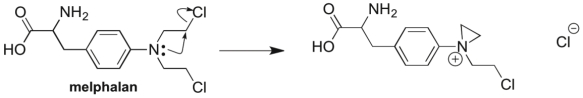
Correct Answer

verified
Nucleophil...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Will the following cation undergo rearrangement?
SHAPE \* MERGEFORMAT 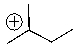

Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a heterolytic bond cleavage, ____ are formed.
A) ions
B) radicals
C) only cations
D) only anions
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the sequence of curved arrows (electron movement) in the steps of the following reaction. 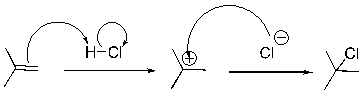
A) proton transfer, proton transfer
B) proton transfer, loss of leaving group
C) nucleophilic attack, proton transfer
D) proton transfer, nucleophilic attack
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would you expect to have the most negative ∆S?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following describes the effect of a catalyst on a reaction?
A) It lowers the free energy of the products.
B) It makes the reactants less stable.
C) It changes the equilibrium constant.
D) It lowers the energy of activation.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Draw curved arrows for each step of the following reaction. 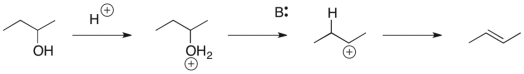
Correct Answer

verified
 _TB4454_00 6.10 Desc...
_TB4454_00 6.10 Desc...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following mechanistic steps represents a nucleophilic attack? 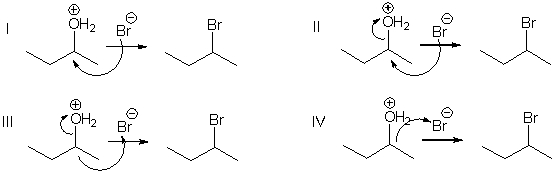
A) I
B) II
C) III
D) IV
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following energy diagrams is of a reaction with one transition state?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the electrophilic site in the following molecule, CH3CH2NHCH2CH3.
A) H
B) N
C) CH2
D) CH3
E) there is no electrophilic site
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Identify the nucleophilic and electrophilic sites in the substrate and reagent of the following reaction. 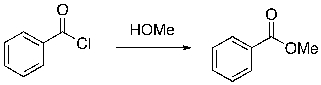
Correct Answer

verified
 _TB4454_00 6.10 Desc...
_TB4454_00 6.10 Desc...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Identify the electrophilic site in the following molecule. 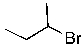
Correct Answer

verified
SHAPE \* M...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following energy diagrams is of a reaction with one intermediate?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using Table 6.1, which of the following is the enthalpy change of the following reaction under standard conditions? 
A) -8 kJ/mol
B) +8 kJ/mol
C) -506 kJ/mol
D) +63 kJ/mol
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 96
Related Exams