A) Na2SO4
B) KNO3
C) Ba SO4
D) Li2CO3
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds behaves as an acid when dissolved in water?
A) CH3OCH3
B) CH4
C) H2SO3
D) KOH
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of an AlBr3 solution if 150.mL of the solution contains 250.mg of Br- ion?
A) 6.95 × 10-3 M
B) 2.08 × 10-2 M
C) 1.67 M
D) 6.23 × 10-3 M
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
By analogy with the oxoacids of sulfur,H2TeO3 would be named
A) hydrotellurous acid.
B) pertelluric acid.
C) telluric acid.
D) tellurous acid.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds behaves as an acid when dissolved in water?
A) SrO
B) C3H8
C) HCl
D) LiOH
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write a balanced net ionic equation for the reaction of AgNO3(aq) with Cu(s) .
A) AgNO3(aq) + Cu(s) → Ag(s) + CuNO3(aq)
B) Ag+(aq) + Cu(s) → Ag(s) + Cu+(aq)
C) 2 AgNO3(aq) + Cu(s) → 2 Ag(s) + CuNO3(aq)
D) 2Ag+(aq) + Cu(s) → 2 Ag(s) + Cu2+(aq)
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When dissolved in water,of HClO4, NH3,KOH,HI,and CH3OH which are acids?
A) NH3 and KOH
B) HClO4 and HI
C) only HI
D) only KOH
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of compounds is soluble in water?
A) Hg2Br2 and Hg2I2
B) CuS and Na2S
C) LiI and Ca(NO3) 2
D) K NO3 and CaCO3
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of BaCl2 are formed when 35.00 mL of 0.00237 M Ba(OH) 2 reacts with excess Cl2 gas? 2 Ba(OH) 2(aq) + 2 Cl2(g) → Ba(OCl) 2(aq) + BaCl2(s) + 2 H2O(l)
A) 0.00864 g
B) 0.0173 g
C) 0.0346 g
D) 0.0829 g
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the following portion of the activity series for oxidation half-reactions,determine which combination of reactants will result in a reaction. Li(s) → Al3+(aq) + e- Cr(s) → Al3+(aq) + 3e-
A) Li(s) with Al(s)
B) Li(s) with Al3+(aq)
C) Li+(aq) with Al3+(aq)
D) Li+(aq) with Al(s)
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
The oxidation number of hydrogen in CaH2 is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number change for the iron atom in the following reaction? 2 Fe2O3(s) + 3 C(s) → 4 Fe(s) + 3 CO2(g)
A) -6
B) -3
C) +3
D) +6
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Glucose,C6H1206,can be represented by the molecular model shown below.If 1.00 mol of glucose is submitted to combustion analysis,how many moles of CO2 and how many moles of H2O would be formed? 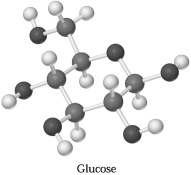
A) 1.00 mol CO2 and 2.00 mol H2O
B) 6.00 mol CO2 and 6.00 mol H2O
C) 6.00 mol CO2 and 12.0 mol H2O
D) 12.0 mol CO2 and 12.0 mol H2O
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Three different substances,A2X,A2Y,and A2Z,were dissolved in water with the following results.(Water molecules are omitted for clarity. ) Which of the substances is the strongest electrolyte,and which is the weakest? 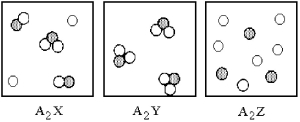
A) A2X is the strongest electrolyte and A2Y is the weakest electrolyte.
B) A2Y is the strongest electrolyte and A2X is the weakest electrolyte.
C) A2Y is the strongest electrolyte and A2Z is the weakest electrolyte.
D) A2Z is the strongest electrolyte and A2Y is the weakest electrolyte.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An aqueous solution of H Cl is named
A) hydrochloric acid.
B) hydrochlorous acid.
C) chloric acid.
D) chlorous acid.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many milliliters of a 6.0 M HNO3 solution are needed to make 0.25 L of a 3.5 M HNO3 solution?
A) 686 mL
B) 428 mL
C) 146 mL
D) 119 mL
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of HCl in the final solution when 65 mL of a 6.0 M HCl solution is diluted with pure water to a total volume of 0.15 L?
A) 1.4 × 10-2 M
B) 2.6 M
C) 14 M
D) 2.6 × 103 M
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of the sulfur atom in S8?
A) -2
B) 0
C) +6
D) +8
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume of a 0.540 M NaOH solution contains 13.5 g of NaOH?
A) 0.182 L
B) 0.625 L
C) 1.60 L
D) 5.49 L
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
What are the two products of the reaction H3PO4(aq)+ 3 KOH(aq)→ ?
Correct Answer

verified
Correct Answer
verified
Showing 161 - 180 of 211
Related Exams